Longevity Conferences 2023
Curated list of Longevity Conferences, where you can explore the latest research and developments in the field of aging and longevity.
Influencing proteostasis pathways might slow age-related decline and prevent diseases, such as Alzheimer's, type 2 diabetes or cystic fibrosis. Proteostasis can be improved through lifestyle changes.

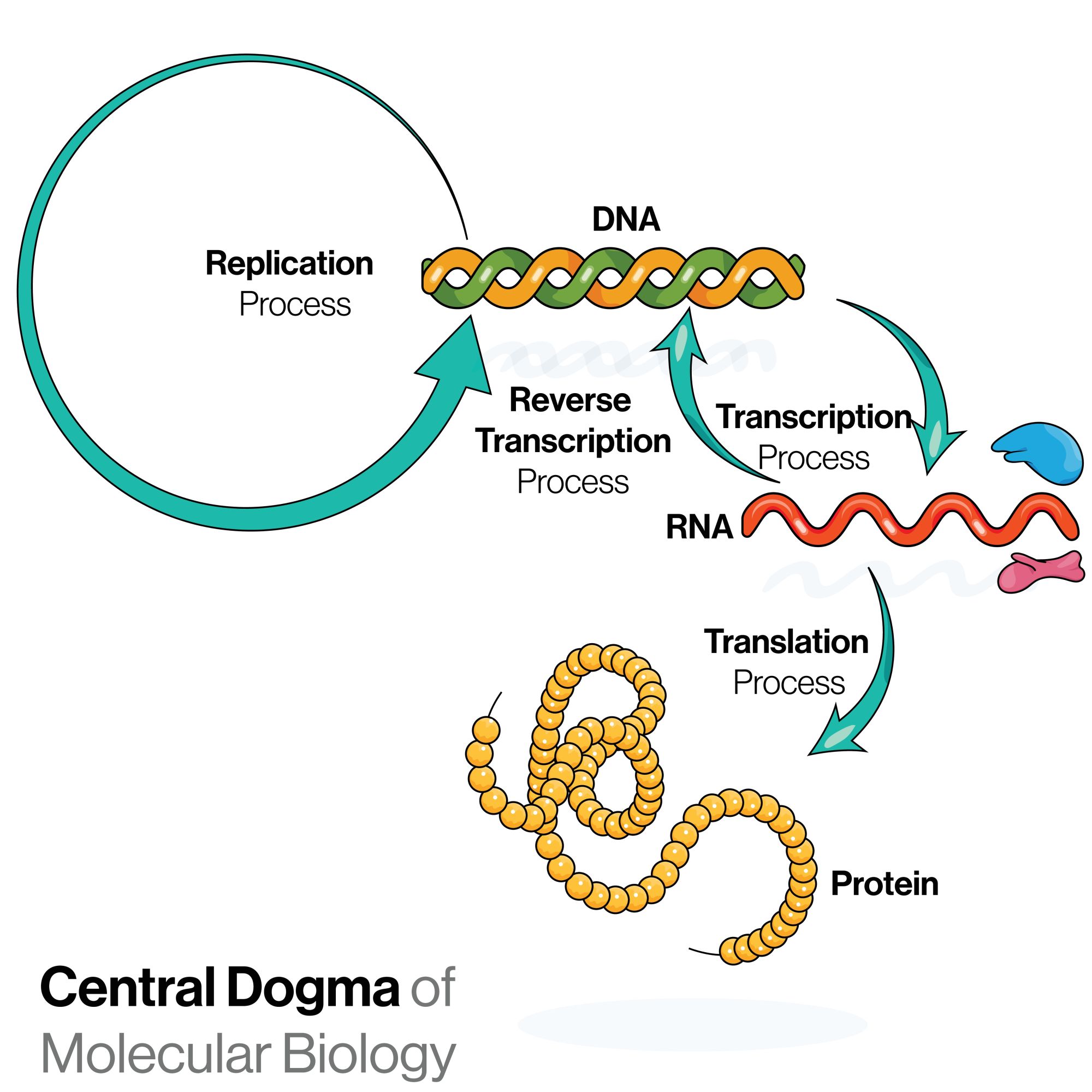
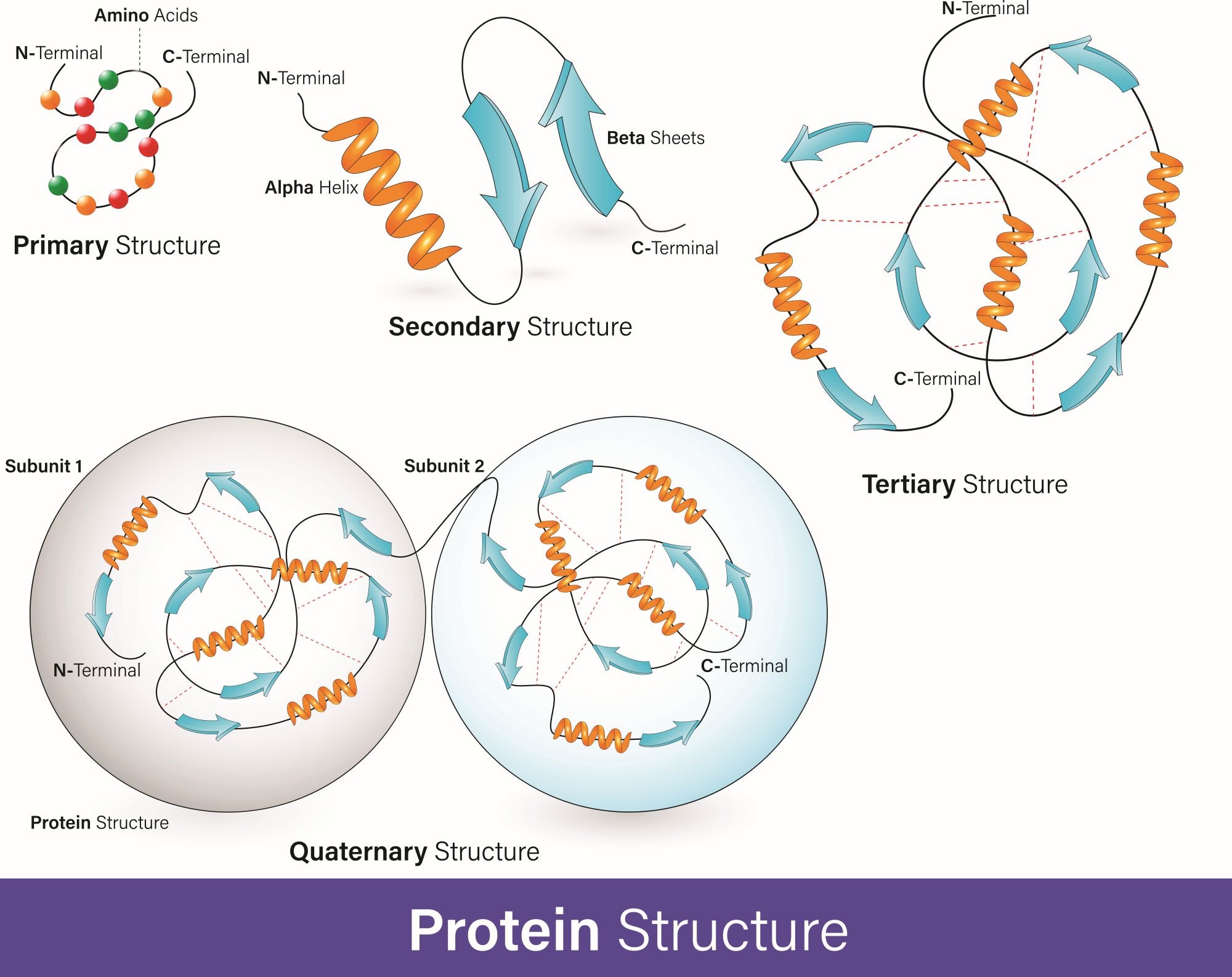
Human cells contain more than 10,000 proteins that convey an incredible variety of biological functions. Most of these proteins must fold into particular 3D structures in order to perform these functions. Through a process called proteostasis, eukaryotic organisms (organisms whose cells have a membrane-bound nucleus) maintain the integrity of protein structures. Proteostasis is a complex system of precisely coordinated mechanisms that rapidly correct unwanted protein changes. Disturbances and failures in this system are considered to be one of the hallmarks of aging. They lead to an accumulation of intracellular damage, which can consequently cause multiple diseases.

The proteome – the entire protein complement expressed by a cell, tissue, or organism – of humans is incredibly complex, with its exact composition varying between different cells and tissues. Protein homeostasis or proteostasis refers to a state of the balanced functioning proteome. In healthy cells, this balance is achieved through a range of mechanisms called proteostasis network, which regulate protein synthesis, structure formation, and degradation. Through this network, proteostasis influences cellular function and enables cells to regulate their physiology for organism development and aging (1).
Proteostasis regulation starts already during translation – a process in which information contained in messenger RNA (mRNA) guides the addition of amino acids to protein structure. During their synthesis, proteins must attain their defined 3D structure through a process known as protein folding. The folded state of a protein is thermodynamically favorable, and folding was initially thought to occur spontaneously. Studies have shown that most proteins (especially complex ones) require special "helper" proteins to fold. These “helpers” are known as molecular chaperones (2). Chaperones aid folding through several mechanisms that might or might not involve ATP (adenosine triphosphate, an energy-carrying molecule), as well as prevent protein aggregation.
Though the folded structure of a protein is energetically favorable, it does not mean that it is always stable. A substantial proportion of protein molecules present in the tissue might be in a partially or entirely unfolded state. This is influenced by many additional factors, such as mutations or external stress (e.g., heightened temperatures, reactive oxygen species, or the presence of heavy metals) (3). Proteins in these unfolded states (or non-native conformations) tend to aggregate, thus losing their functionality.
Regulated degradation is another critical part of protein homeostasis, which helps avoid an accumulation of faulty or misfolded proteins, and adapts protein levels depending on the environment (4). Two major pathways participate in protein degradation – the ubiquitin system (5) and autophagy (6). Both degradation systems use molecular chaperones to help detect misfolded and defective proteins.
Although the proteostasis network is robust, it is still prone to errors. Despite the abundant number of chaperones, protein synthesis and folding have been estimated to result in 5-30% of improperly folded proteins, which must be promptly degraded (7). Besides, defective mRNAs, causing incomplete protein chains, or lack of transport RNA could also lead to faulty proteins. This, in turn, may limit the rate of translation resulting in protein aggregation (8,9).
Stress conditions, such as oxidative stress, heat stress, or toxic agents (10), can lead to protein unfolding (which increases aggregating risk) and require the employment of protein degradation machinery.
Multiple faults in the proteostasis network can be amended by targeted protein degradation, which is regulated by the already-mentioned molecular chaperones. An intricate balance between protein synthesis creation and degradation can shift due to genetic reasons (11) or cellular senescence (12). This can lead to gradual loss of proteostasis.
Accumulating protein aggregates is a culprit in many neurodegenerative conditions, including Alzheimer's and Parkinson's. However, those are not the only conditions affected by proteostasis. The role of protein misfolding is now widely recognized in such disorders as type 2 diabetes (13) and cystic fibrosis (14).
Generally, diseases associated with proteostasis disruptions can be classified as loss- or gain-of-function disorders (15). Loss-of-function diseases (such as cystic fibrosis) are linked to inherited mutations. In contrast, gain-of-function (such as Alzheimer's disease, Huntington's disease, and lateral sclerosis) are often caused by excessive accumulation of protein aggregates. Though the exact mechanisms are not yet completely understood, protein aggregates are well-known to cause toxicity, cellular dysfunction, and eventual cell death. Two principal reasons behind these are, at the moment, thought to be:
Many of the abovementioned conditions fall into the category of age-related diseases. And age-related changes in the proteome are known to be one of the key drivers of aging (16). As we age, the balance between protein synthesis and degradation worsens, and folding mechanisms' functionality declines.
During aging, the proteostasis network faces the increasing load of misfolded proteins, proteins damaged by oxidative stress (17), and the repressed expression of chaperones due to a lack of ATP production (18). Once the proteostasis drops below a critical level, aggregation can no longer be controlled. An imbalance can be additionally worsened by the additional forms of stress, creating a feedback loop that causes further protein aggregation (19). This can lead to complete proteostasis collapse.
However, a collapse is not inevitable, and a range of findings point toward that. Evidence suggests that proteostasis is controlled by the same signaling pathways that influence longevity (20–22), at least in model organisms. For example, inhibition of the insulin signaling pathway in nematodes (the major lifespan-extending manipulation in this species) results in a significant upregulation of the chaperone network. And vice versa, improvement in proteostasis was noticed to influence the lifespan of model organisms. Also, not all cells suffer equally from age-dependent proteostasis loss. For example, it has been demonstrated that stem cells tend to exhibit elevated levels of misfolded proteins degrading (23,24).
Insights from proteome changes occurring during aging and disease progression can provide strategies to improve proteostasis. Several treatments are emerging aimed at restoring the proteostasis network and delaying the onset of age-related disorders, as well as influencing longevity.
One of the current approaches toward loss-of-function diseases is direct intravenous administration of a functional version of a protein to restore the normal function of a defective protein (25,26). Though this approach proved effective for some disorders (such as Alzheimer’s disease), alternative approaches are needed as a low percentage of the injected protein makes it to the target part of the cell. Also, protein replacement is generally not applicable for brain disorders as proteins are unable to cross the blood-brain barrier.
Protein stabilization uses either so-called pharmacological chaperone proteins (27) or small molecules (28) that bind to and stabilize a functional form of a target protein. This approach is promising both for gain- or loss-of-function disorders. Both methods have been proven to be effective in clinical trials for transthyretin amyloid disease (a disorder in which protein aggregates are progressively accumulated in the heart), neuropathy (damage or dysfunction of nerves), and cardiomyopathy (disease linked to the weakening of the heart muscle).
In this more general variation of the previous approach, the innate biology of the cell is readapted through the introduction of proteostasis regulators (RNA, DNA, small molecules, or proteins) (29,30). The aim here is to alter the proteostasis network itself at any chosen step by manipulating signaling pathways. Unlike in the previous approach, proteostasis regulators are able to improve several conditions at once by enhancing the general efficacy of the proteostasis network.
All these approaches are currently being actively developed and tested. A growing body of evidence suggests that proteostasis regulators, in particular, are able to correct deficiencies that contribute to a broad range of human diseases and influence aging.
Ways to improve and influence proteostasis are currently being studied, so the number of tips you can give to your clients is somehow limited. Below you will find several points you can address:
However, there are no precise recommendations regarding proteostasis at the moment, which must also be kept in mind.
Proteome balance maintenance is challenging for our organisms, especially in the face of aging. The research shows that, by influencing the innate proteostasis pathways, we might be able to slow age-related decline and prevent disease. Novel possibilities of pharmacological intervention offer great promise at fighting diseases now seen as incurable and prolonging human healthspan.
Human cells contain more than 10,000 proteins that convey an incredible variety of biological functions. Most of these proteins must fold into particular 3D structures in order to perform these functions. Through a process called proteostasis, eukaryotic organisms (organisms whose cells have a membrane-bound nucleus) maintain the integrity of protein structures. Proteostasis is a complex system of precisely coordinated mechanisms that rapidly correct unwanted protein changes. Disturbances and failures in this system are considered to be one of the hallmarks of aging. They lead to an accumulation of intracellular damage, which can consequently cause multiple diseases.

The proteome – the entire protein complement expressed by a cell, tissue, or organism – of humans is incredibly complex, with its exact composition varying between different cells and tissues. Protein homeostasis or proteostasis refers to a state of the balanced functioning proteome. In healthy cells, this balance is achieved through a range of mechanisms called proteostasis network, which regulate protein synthesis, structure formation, and degradation. Through this network, proteostasis influences cellular function and enables cells to regulate their physiology for organism development and aging (1).
Proteostasis regulation starts already during translation – a process in which information contained in messenger RNA (mRNA) guides the addition of amino acids to protein structure. During their synthesis, proteins must attain their defined 3D structure through a process known as protein folding. The folded state of a protein is thermodynamically favorable, and folding was initially thought to occur spontaneously. Studies have shown that most proteins (especially complex ones) require special "helper" proteins to fold. These “helpers” are known as molecular chaperones (2). Chaperones aid folding through several mechanisms that might or might not involve ATP (adenosine triphosphate, an energy-carrying molecule), as well as prevent protein aggregation.
Though the folded structure of a protein is energetically favorable, it does not mean that it is always stable. A substantial proportion of protein molecules present in the tissue might be in a partially or entirely unfolded state. This is influenced by many additional factors, such as mutations or external stress (e.g., heightened temperatures, reactive oxygen species, or the presence of heavy metals) (3). Proteins in these unfolded states (or non-native conformations) tend to aggregate, thus losing their functionality.
Regulated degradation is another critical part of protein homeostasis, which helps avoid an accumulation of faulty or misfolded proteins, and adapts protein levels depending on the environment (4). Two major pathways participate in protein degradation – the ubiquitin system (5) and autophagy (6). Both degradation systems use molecular chaperones to help detect misfolded and defective proteins.

Although the proteostasis network is robust, it is still prone to errors. Despite the abundant number of chaperones, protein synthesis and folding have been estimated to result in 5-30% of improperly folded proteins, which must be promptly degraded (7). Besides, defective mRNAs, causing incomplete protein chains, or lack of transport RNA could also lead to faulty proteins. This, in turn, may limit the rate of translation resulting in protein aggregation (8,9).
Stress conditions, such as oxidative stress, heat stress, or toxic agents (10), can lead to protein unfolding (which increases aggregating risk) and require the employment of protein degradation machinery.
Multiple faults in the proteostasis network can be amended by targeted protein degradation, which is regulated by the already-mentioned molecular chaperones. An intricate balance between protein synthesis creation and degradation can shift due to genetic reasons (11) or cellular senescence (12). This can lead to gradual loss of proteostasis.
Accumulating protein aggregates is a culprit in many neurodegenerative conditions, including Alzheimer's and Parkinson's. However, those are not the only conditions affected by proteostasis. The role of protein misfolding is now widely recognized in such disorders as type 2 diabetes (13) and cystic fibrosis (14).
Generally, diseases associated with proteostasis disruptions can be classified as loss- or gain-of-function disorders (15). Loss-of-function diseases (such as cystic fibrosis) are linked to inherited mutations. In contrast, gain-of-function (such as Alzheimer's disease, Huntington's disease, and lateral sclerosis) are often caused by excessive accumulation of protein aggregates. Though the exact mechanisms are not yet completely understood, protein aggregates are well-known to cause toxicity, cellular dysfunction, and eventual cell death. Two principal reasons behind these are, at the moment, thought to be:
Many of the abovementioned conditions fall into the category of age-related diseases. And age-related changes in the proteome are known to be one of the key drivers of aging (16). As we age, the balance between protein synthesis and degradation worsens, and folding mechanisms' functionality declines.
During aging, the proteostasis network faces the increasing load of misfolded proteins, proteins damaged by oxidative stress (17), and the repressed expression of chaperones due to a lack of ATP production (18). Once the proteostasis drops below a critical level, aggregation can no longer be controlled. An imbalance can be additionally worsened by the additional forms of stress, creating a feedback loop that causes further protein aggregation (19). This can lead to complete proteostasis collapse.
However, a collapse is not inevitable, and a range of findings point toward that. Evidence suggests that proteostasis is controlled by the same signaling pathways that influence longevity (20–22), at least in model organisms. For example, inhibition of the insulin signaling pathway in nematodes (the major lifespan-extending manipulation in this species) results in a significant upregulation of the chaperone network. And vice versa, improvement in proteostasis was noticed to influence the lifespan of model organisms. Also, not all cells suffer equally from age-dependent proteostasis loss. For example, it has been demonstrated that stem cells tend to exhibit elevated levels of misfolded proteins degrading (23,24).

Insights from proteome changes occurring during aging and disease progression can provide strategies to improve proteostasis. Several treatments are emerging aimed at restoring the proteostasis network and delaying the onset of age-related disorders, as well as influencing longevity.
One of the current approaches toward loss-of-function diseases is direct intravenous administration of a functional version of a protein to restore the normal function of a defective protein (25,26). Though this approach proved effective for some disorders (such as Alzheimer’s disease), alternative approaches are needed as a low percentage of the injected protein makes it to the target part of the cell. Also, protein replacement is generally not applicable for brain disorders as proteins are unable to cross the blood-brain barrier.
Protein stabilization uses either so-called pharmacological chaperone proteins (27) or small molecules (28) that bind to and stabilize a functional form of a target protein. This approach is promising both for gain- or loss-of-function disorders. Both methods have been proven to be effective in clinical trials for transthyretin amyloid disease (a disorder in which protein aggregates are progressively accumulated in the heart), neuropathy (damage or dysfunction of nerves), and cardiomyopathy (disease linked to the weakening of the heart muscle).
In this more general variation of the previous approach, the innate biology of the cell is readapted through the introduction of proteostasis regulators (RNA, DNA, small molecules, or proteins) (29,30). The aim here is to alter the proteostasis network itself at any chosen step by manipulating signaling pathways. Unlike in the previous approach, proteostasis regulators are able to improve several conditions at once by enhancing the general efficacy of the proteostasis network.
All these approaches are currently being actively developed and tested. A growing body of evidence suggests that proteostasis regulators, in particular, are able to correct deficiencies that contribute to a broad range of human diseases and influence aging.

Ways to improve and influence proteostasis are currently being studied, so the number of tips you can give to your clients is somehow limited. Below you will find several points you can address:
However, there are no precise recommendations regarding proteostasis at the moment, which must also be kept in mind.
Proteome balance maintenance is challenging for our organisms, especially in the face of aging. The research shows that, by influencing the innate proteostasis pathways, we might be able to slow age-related decline and prevent disease. Novel possibilities of pharmacological intervention offer great promise at fighting diseases now seen as incurable and prolonging human healthspan.

